Which of the Following Best Describes a Single Replacement Reaction
Which reaction type best describes the. Which of the statements below best describes the following reaction.

Chemical Formula Writing Simplified Writing Worksheets Chemical Formula Writing
An essay that includes a synthesis of two sources as well as the authors opinion is a synthesis and response essay.

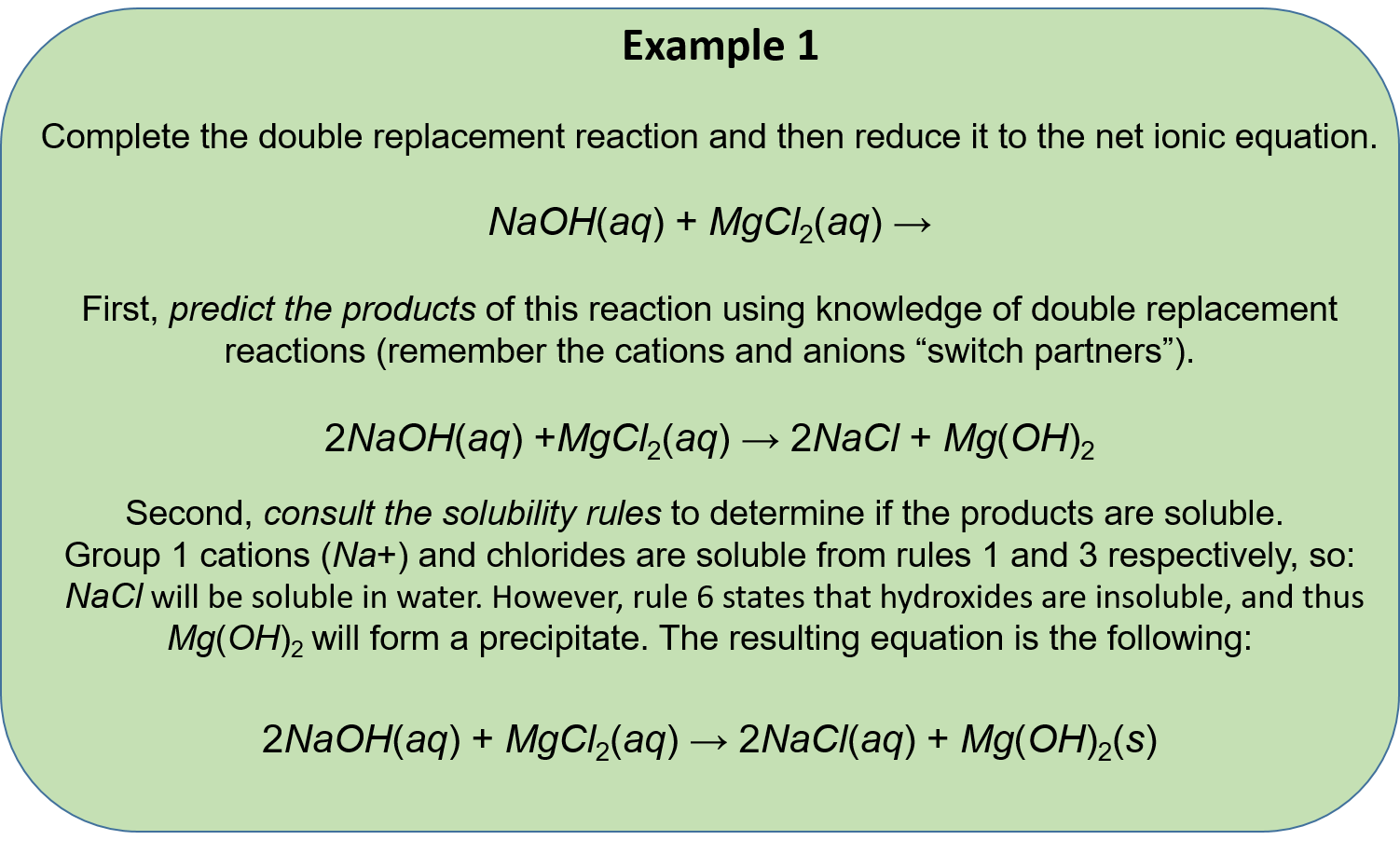
. This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places. 1 See answer Advertisement Advertisement laurenkropp is waiting for your help. D all of the above.
Two elements combine to form a compound one element takes the place of another in a compound two elements switch places in a compound a compound breaks into separate elements. Balance polyatomic ions as a single unit check each reactant and product to verify the coefficients. Zns CuSO4aq - Cu and ZnSO4.
Na s H 2 O l. Which of the following best describes a single-replacement reaction. Hydrogen usually forms the cation in a single replacement reaction.
The common non-metals in single replacement reactions are the group 17 elements which generally form anions with a 1- charge. The the following reaction is. F H2 I2 2HI G 2NaCl 2Na Cl2 H NaF HCl HF NaCl.
What best describes a synthesis and response essay. Sodium metal reacts with water in the following single replacement reaction. This is the best answer based on feedback and ratings.
How much heat is needed for this total process. See the answer See the answer done loading. Which of these reactions shows simple chemical decomposition.
F H2SO4 CaOH2 CaSO4 H2O. E none of the above. 2 question The reaction 2Mgs O2g -- 2MgOs is a Group of answer choices double-replacement reaction.
A compound breaks apart into separate elements C. Mg 2HCl MgCl2 H2. HCl aq KOH aq KCl aq H2O l A Aqueous solutions of hydrochloric acid and potassium hydroxide react.
One element takes the place of another in a compound. One element takes the place of another in a compound B. C A flame is observed.
Which of the following best represents the reaction between sulfuric acid and calcium hydroxide. Add your answer and earn points. The reaction above is classified as.
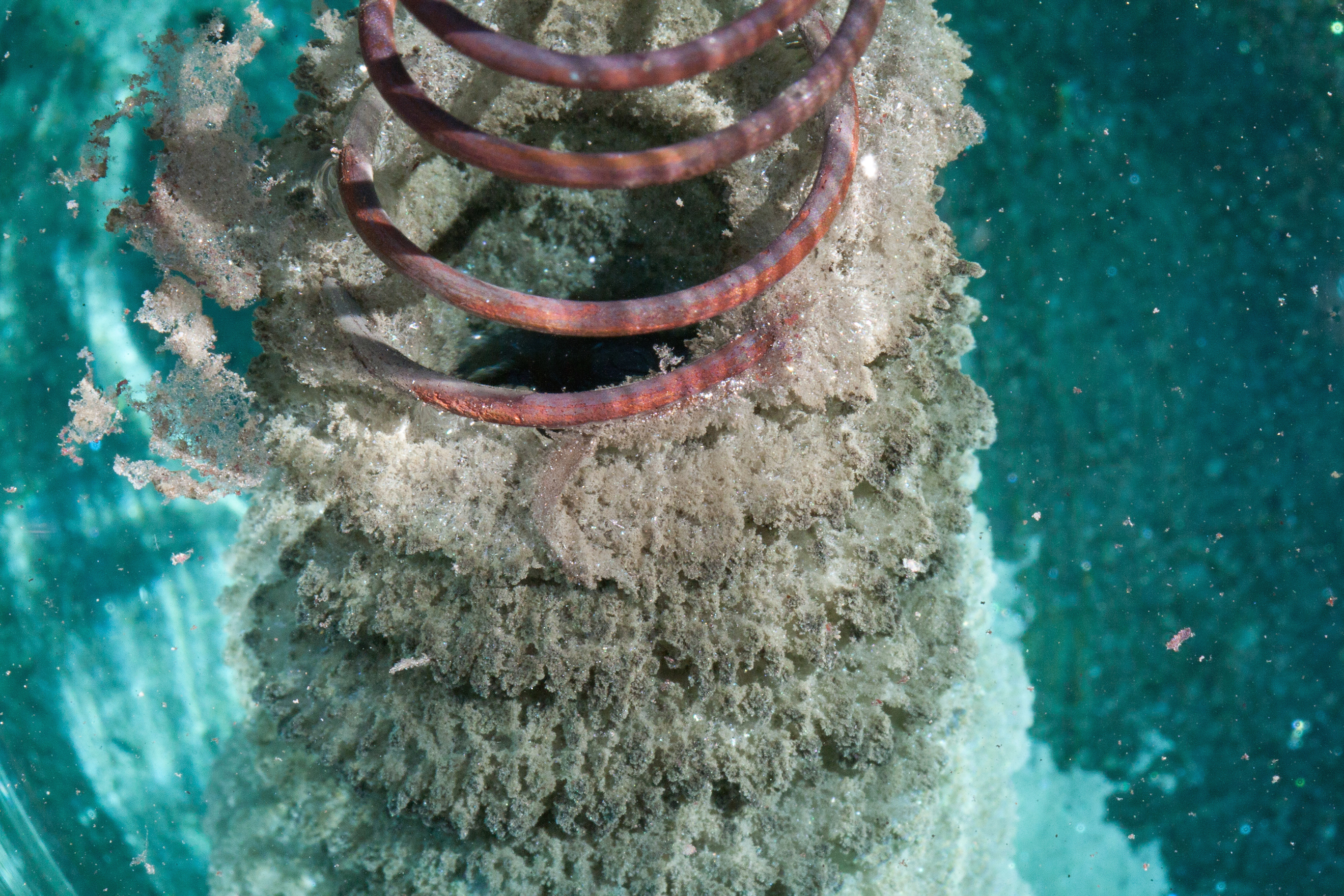
MathrmZns2 mathrmHCla q rightarrow mathrmZnCl_2a qmathrmH_2g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2 lowering the temperature and using a single piece of zinc rather. Because Magnesium Replaces Hydrogen. C Single replacement D Combustion.
Which of the following is a single replacement reaction. Well its D Mg 2HCl ----- MgCl2 H2. B A gas is detected.
HINT you will need to perform two calculations and then add up the results. All of the above. Which of the following describes a single-replacement reaction.
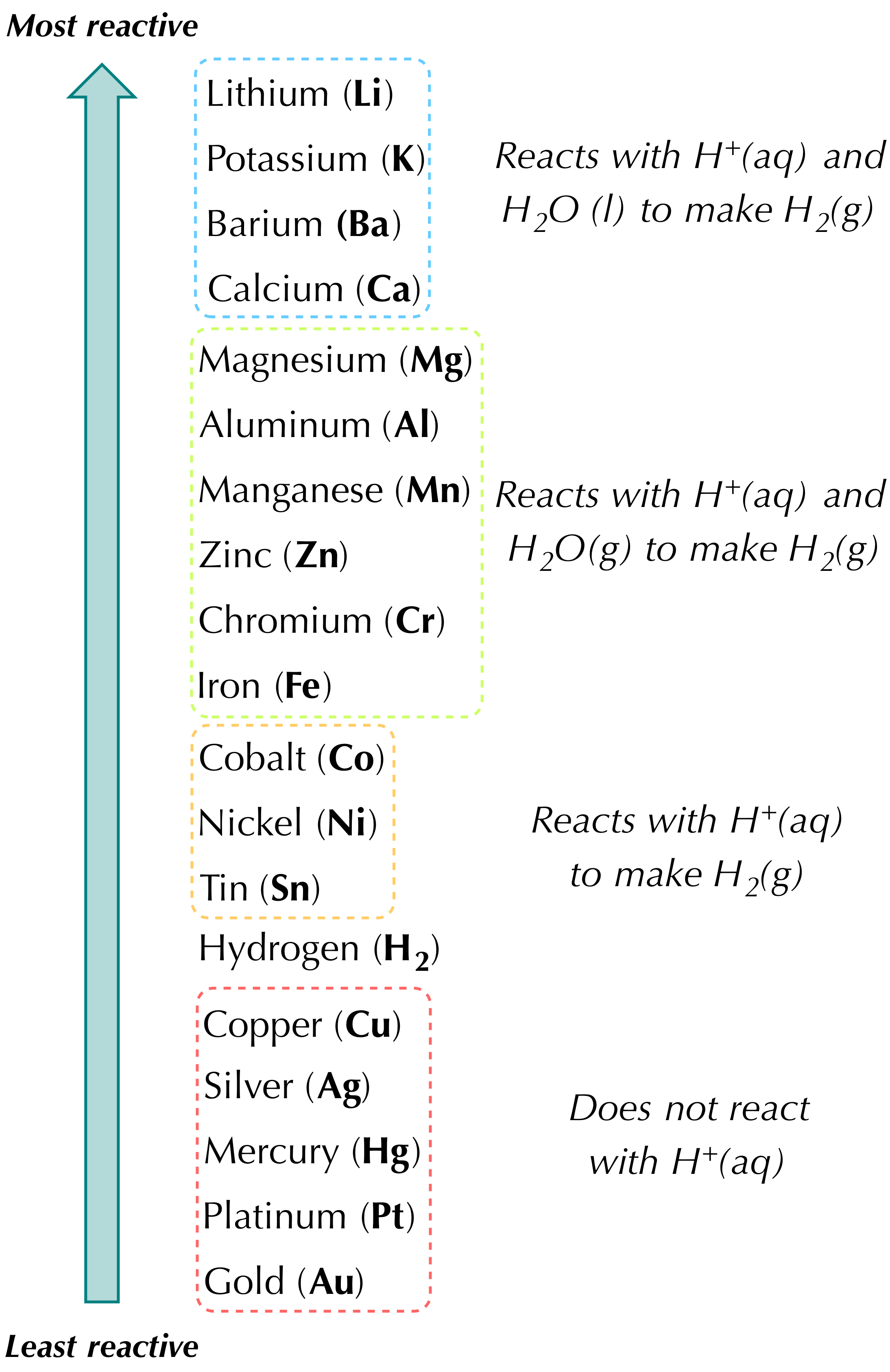
See answers 2 Best Answer. A A precipitate is formed. The three general categories of single-replacement reactions are.
Two elements switch places in. The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine. 2 When magnesium is burned in the presence of oxygen it produces magnesium oxide according to the following chemical equation.
NaOH aq H 2 g Determine the limiting reactant and mass of hydrogen gas produced when 20g of sodium is added to 100g of water. Consider the following equation. Which of the statements below best describes the following reaction.
Simpler compounds from a complex compound. In other words it is where an element reacts with a compound and replaces one component of the compound. Ice with a mass of 15 grams is melted AND then the temperature of the now liquid water is raised from 0 degrees Celsius up to 95 degree Celsius.
While the Sodium ion on. Dall of the above. Two elements combine to form a compound D.
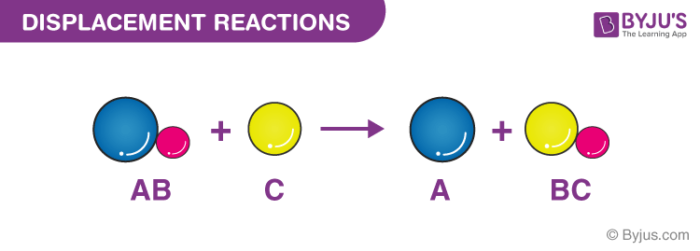
Which of the following best describes a single replacement reaction. A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species. BaCO3 -- BaO CO2.
What are the products from the following single-replacement reaction.

This Is A Classic Example Of A Single Replacement Chemical Reaction Wherein Copper Ions Precipitate Out From Soluti Chemistry Labs Medical Mnemonics Chemistry

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
Ch104 Chapter 5 Chemical Reactions Chemistry

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
What Are Some Examples Of Displacement Reactions In Chemistry Quora

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube

A Mole Ratio Is The Ratio Amounts Of The Entities In A Chemical Reaction Chemistry Worksheets Chemistry Lessons Teaching Chemistry

My First Ipad Note Post I Ve Been Using Notability Almost Religiously It S My Favorite App For

Combustion Reactions Chemical Reactions Chemical Equation Reactions

Double Replacement Reaction Is A Type Of Chemical Reaction Where Two Compounds React And The Positive Negative I Positive And Negative Positivity Negative Ions
Displacement Reactions Definition Types Single Double Examples
Single Replacement Reactions Article Khan Academy
Single Replacement Reactions Article Khan Academy
What Are Some Examples Of Displacement Reactions In Chemistry Quora

Home Annmarie Molina Positive Quotes For Life Inspirational Quotes Motivation Inspirational Quotes

Single Replacement Reaction Definition And Examples



Comments
Post a Comment